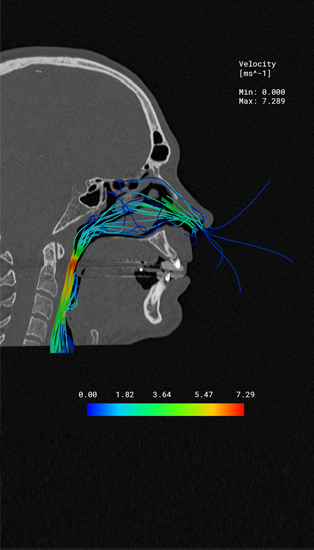
Computer-supported simulation is revolutionizing medical technology by precisely representing the behaviour of medical devices in the virtual patient - even during development.
Arrange a consultationUsing Simq for numerical simulation in medicine and medical technology.
By means of simulation, complex processes and loads in the patient can be represented virtually - in silico. This allows you to significantly reduce the time required for clinical studies in all test and approval phases. Simq's simulation technologies, which can be used in the medical environment, reduce costs and will give your projects a time advantage.
Numerical simulation in the approval process of medical devices
Leap in development
In silico testing - a milestone in future medical device development
Computer modelling and simulation have proven their worth in highly complex and critical technology sectors such as aerospace, chip design or power engineering. These reliable simulation methods have the potential to revolutionize the approval of medical devices by reliably replacing in vitro and in vivo testing within silico. In the future, the use of unethical animal and human experiments will be shifted to the level of virtual test set-ups including virtual patients. Thus offering the possibility of testing and adapting product developments safely at an early stage. Accelerated approval of medical products will then become possible and can also be scheduled.
Digital Verification
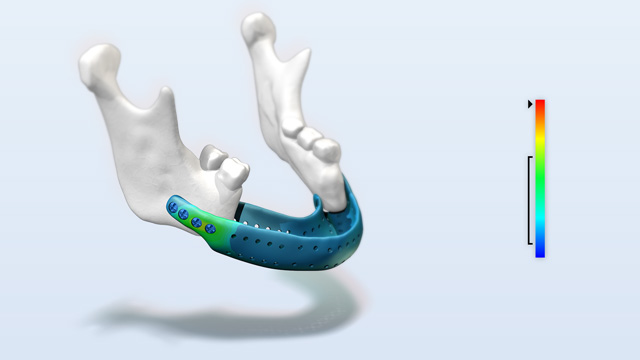
Strength assessment of patient specific implants
The reconstruction of a jaw after tumor resections is a great challenge. For this purpose, patient-specific implants are often manufactured. Until now, these implants have only been designed based on experience and could not be tested. With the docq VIT software, developed by Simq, it is possible to illustrate and prove the safety and performance of patient-specific medical devices, e.g. implants, under realistic loads with little effort.
Simulation technology for medicine with Simq
As a competent project and service partner, Simq supports and accompanies its customers in all questions concerning the preparation, planning and implementation of numerical software in medical technology companies. Our services are certified according to ISO 13485. Highly specialized and as an independent sister company of CADFEM, we serve many well-known customers in the field of medicine and medical technology. Please visit simq.de for further information or contact Simq directly.
Consulting

We offer individual company workshops on site at the customer's premises, in which we jointly identify the best possible applications for simulation in the company.
Project support
Within the scope of project support, we show how simulation can be used most efficiently in product development and approvals.
Further training
Simq has a specialized seminar which offer regular events.
Simulation on demand

We support you in using hardware and licenses on a project-specific basis and scaling as needed.
Customer References